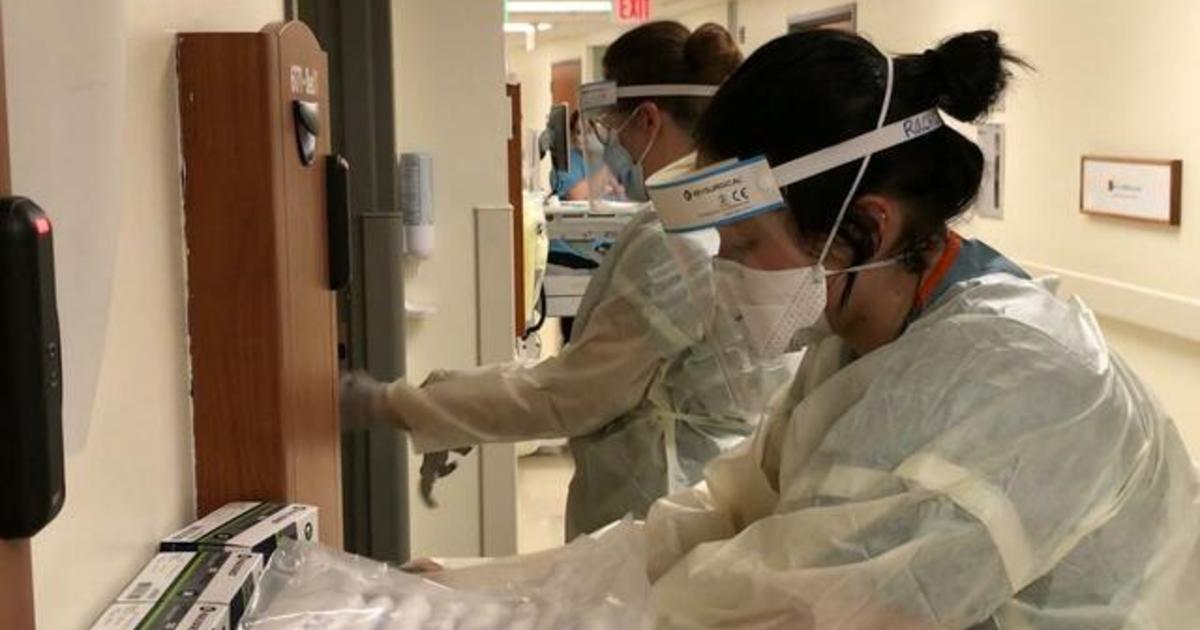
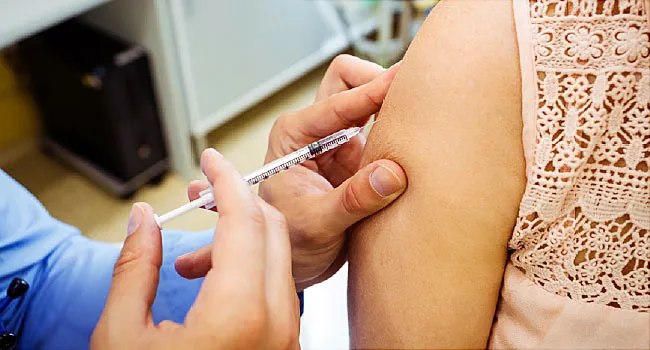
There’s no proof yet to recommend that a Covid-19 vaccine booster shot is required, a working group for the Centers for Illness Control and Avoidance stated Wednesday.
That might alter as the pandemic progresses, nevertheless, and public health authorities will continue to keep track of the infection to identify if extra shots are called for in the future.
Complete protection of the coronavirus break out
Members of an independent group of consultants to the CDC, called the Advisory Committee on Immunization Practices, concurred with the working group’s conclusion.
There are a variety of tough concerns on the subject of boosters, however, consisting of the requirement to cover unvaccinated individuals in the U.S. and the rest of world with a very first dosage prior to improving totally immunized people.
” Previous to walking around and offering everybody boosters, we require to enhance the total vaccination rate,” stated a member of the committee, Dr. Keipp Talbot, an associate teacher of medication at Vanderbilt University.
Another concern presented to the advisory committee is what proof should be utilized to choose whether boosters are required?
A clear indication would be increasing cases in completely immunized people. If particular surrogate procedures– like subsiding antibody or T cell levels– might be determined, boosters might possibly be offered to avoid an increase in cases.
It’s still uncertain what those cutoff levels, called correlates of defense, may be, Dr. Sharon Frey, scientific director of the Center for Vaccine Advancement at Saint Louis University Medical School, stated.
” I believe the only thing we can do at this minute is, if we begin to see an uptick in reinfection in individuals, or brand-new infections in individuals who have actually been immunized, that’s our hint that we require to move rapidly,” Frey stated.
Dr. Jose Romero, the advisory committee’s chair, kept in mind that for influenza vaccines, public health authorities do not wait up until influenza is extensive to immunized individuals– however much is learnt about the security of that vaccine.
” We ought to start to think about utilizing these boosters prior to we have proof of illness from these variations, if this continues to be a continuous infection with routine recirculation,” Romero stated, describing the delta version in specific.
In addition to proof of subsiding efficiency, the security of an extra dosage will be assessed, along with the seriousness of any adverse effects, Dr. Doran Fink, deputy director of the Fda’s department of vaccines, stated throughout the conference.
Vaccine boosters
Within weeks of the very first Pfizer-BioNTech and Moderna vaccines entering into individuals’s arms in the United States, both business revealed they were beginning trials taking a look at whether a 3rd dosage would be required.
It stays uncertain what a 3rd dosage would appear like; both Pfizer and Moderna are examining providing a 3rd dosage of a vaccine similar to the very first 2, along with a 3rd dosage that’s been modified to much better target specific versions of the coronavirus.
Johnson & Johnson has likewise stated it’s studying whether another dosage– in its case, a 2nd dosage– will be required to up defense versus the infection.
The National Institute of Allergic Reaction and Contagious Illness, or NIAID, is checking out boosters also, consisting of a scientific trial that research studies whether a 3rd shot of a Moderna vaccine might be offered after an individual at first got 2 shots of Pfizer, or one shot of Johnson & Johnson.
Arise From the Pfizer, Moderna and NIAID research studies are anticipated this summer season, Dr. Sara Oliver, a medical epidemiologist with CDC’s National Center for Immunization and Breathing Illness, stated throughout a discussion to the advisory committee.
Though the existing proof recommends that at this moment, a booster isn’t required, researchers will continue to keep an eye on the information. “A vote to advise boosters, in any population could, take place whenever information support upgraded policy,” Oliver stated.
Proof to concentrate on consists of how well the vaccines operate in susceptible populations, such as long-lasting care citizens, older grownups and immune-compromised clients, she stated.
Download the NBC News app for complete protection of the coronavirus break out
A drop in individuals’s resistance levels– as determined through antibodies or T cells, for instance– or an increase in the rate of advancement infections might indicate that the vaccines’ efficiency is subsiding.
Dr. John Beigel, associate director of medical research study for the department of microbiology contagious illness at the National Institute of Allergic Reaction and Contagious Illness, informed NBC News that scientists there are checking out the correlates of security. This finding will assist scientists in all vaccine trials identify whether an individual’s resistance has actually fallen below needed levels.
Beigel stated he anticipates those information to be readily available in the next 2 months.
Another factor a booster might be required is if a brand-new alternative emerges that averts the vaccines, stated Dr. Dan Barouch, director of the center for virology and vaccine research study at Beth Israel Deaconess Medical Center in Boston.
” That hasn’t took place yet,” Barouch stated, who dealt with establishing the Johnson & Johnson vaccine. He is not a member of the advisory committee.
Any trial taking a look at booster dosage research study will likely concentrate on lab information instead of taking a look at how protective the vaccine remains in the real life, which is what the initial vaccine trials did, Barouch stated. That implies that rather of offering some individuals a booster and others a placebo and after that seeing the number of infections happen in the real life, scientists would rather compare the resistance levels in both groups, to see if individuals who got the booster saw a substantial boost.
Follow NBC HEALTH on Twitter & Facebook
http://dentalassistantclasses.net/no-proof-yet-to-recommend-covid-vaccine-booster-is-required-cdc-group-states/






![author['full_name']](https://clf1.medpagetoday.com/media/images/author/johnGever_188.jpg) < img alt="author['full_name']
" src="https://clf1.medpagetoday.com/media/images/author/johnGever_188 jpg" >
< img alt="author['full_name']
" src="https://clf1.medpagetoday.com/media/images/author/johnGever_188 jpg" >


![author['full_name']](https://clf1.medpagetoday.com/media/images/author/crystalPhend_188.jpg)
 http://dentalassistantclasses.net/the-isolite-and-advantages-for-dental-assistants/
http://dentalassistantclasses.net/the-isolite-and-advantages-for-dental-assistants/

/cloudfront-us-east-2.images.arcpublishing.com/reuters/RUDAPMJHZRJLXLENAYJP2VG2DQ.jpg)



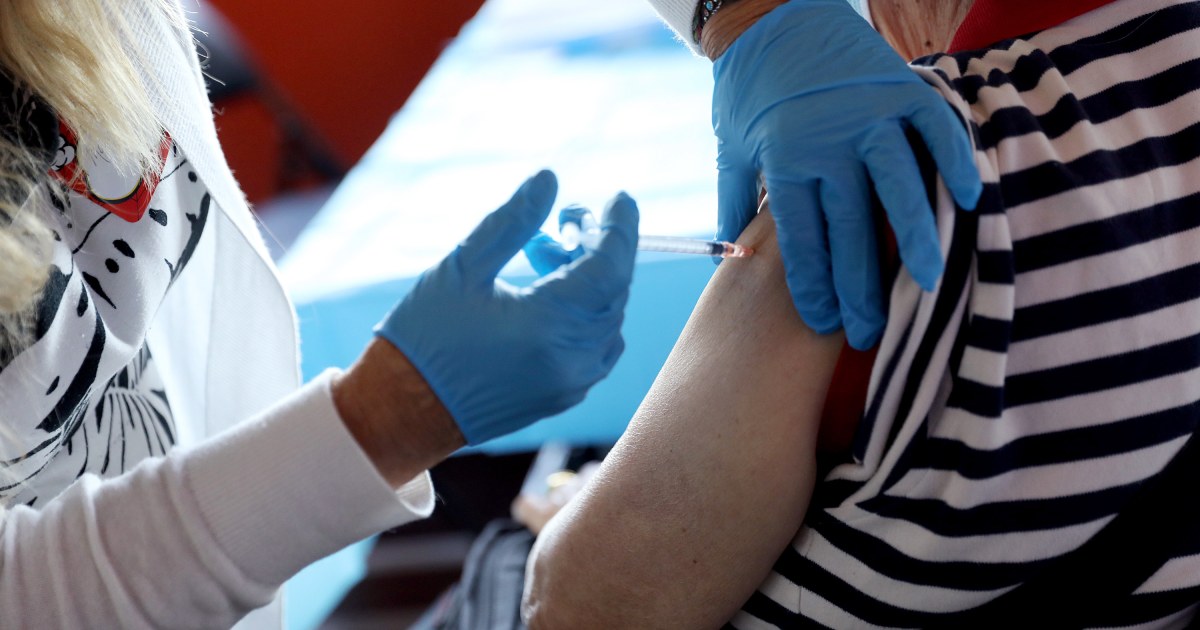


 http://dentalassistantclasses.net/oral-helping-pilbeam/
http://dentalassistantclasses.net/oral-helping-pilbeam/